Protein Complex Characterization Using the iEM Platform
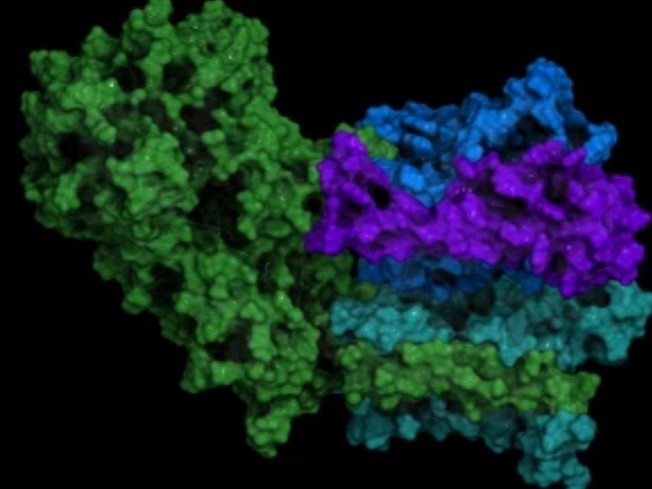
In the past few years, breakthroughs in cryo-electron microscopy (cryo-EM) technology have made it a powerful tool for solving difficult protein structures, including large assemblies, membrane proteins, and multi-protein complexes. Here we provide single-particle cryo-EM to support protein complex characterization, involving their spatial organization and functional modification. Single-particle cryo-EM is a well-established technique in structural biology and can be applied as a complementary tool to traditional structure techniques (such as X-ray crystallography). Based on the advanced technique and experienced experts of various backgrounds, Creative Biostructure is dedicated to supporting the diverse research and application needs of its customers, especially the structure-based drug design projects.
Single-Particle Cryo-EM in Structural Determination of Protein Complexes
Single-particle cryo-EM is the most cutting-edge technology of cryo-EM. This technique enables the 3D structure of macromolecule proteins, which is based on 2D image projection and 3D image modeling analysis. An aqueous biological sample is frozen rapidly and irradiated with a beam of electrons from transmission electron microscopy(TEM). A detector senses how the electrons are scattered and computerized image processing techniques are used to reconstruct the 3D shape of the molecule. Single-particle Cryo-EM not only does not require crystals but also has the ability to analyze specimens with heterogeneous composition and/or conformation. This powerful technique is capable of determining the structure of protein complexes at a 3-5 Å resolution range, with sizes ranging from ~200 kDa to hundreds of megadaltons.
Protein Complex Characterization at the iEM Platform
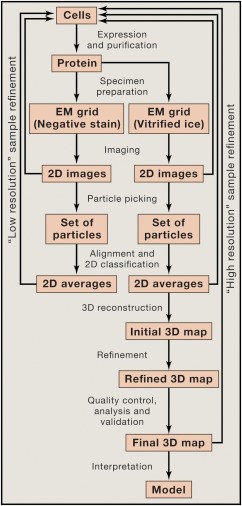 Fig1. The steps involved in structure
Fig1. The steps involved in structure
determination by single-particle cryo-EM.
(Cheng, Y., et al, 2015)
For successful protein complex characterization, providing highly biochemically pure and well-characterized samples is a critical step. To ensure high-resolution 3D reconstruction, we focus on sample preparation, involving sample homogeneity, particle distribution, assembly state, complex size, and so on. In addition, our platform features an improved image digital registration system and algorithms for fast and efficient processing of recorded images as well as subsequent structural analysis, facilitating the determination of structures at near-atomic resolution. Using single-particle cryo-EM, we are capable of solving macromolecular complex structures, such as protein mutants, protein-protein complexes, and protein-nucleic acid complexes.
The workflow of our services, including
Protein purification for single-particle cryo-EM analysis.
Sample preparation for single-particle cryo-EM analysis.
Data acquisition and image processing.
3D reconstruction and atomic model building.
Structure validation and analysis.
Benefits of Our Services
Only micrograms of proteins are required for analysis.
Samples in a fully hydrated, close to the native state.
Heterogeneous samples can be analyzed, allowing the study of dynamic and unstable complexes.
Customized solutions meeting client needs.
Single-particle cryo-EM provides valuable structural information that complements data from other structural biology techniques to help generate a more complete picture of protein complexes. Creative Biostructure is a forward-looking research institute as well as a leading custom service provider in structural biology. We are dedicated to providing the highest quality EM for our global customers. If you are interested in our solutions, please feel free to contact us. Our experienced and professional staff will get back to you as soon as possible.
Cheng, Y., et al. (2015). "A primer to single-particle cryo-electron microscopy." Cell, 161(3), 438-449.
Costa, T. R., et al. (2017). "Structural analysis of protein complexes by cryo electron microscopy." Bacterial Protein Secretion Systems, 377-413.

