Drug Foreign Particle Analysis Using the iEM Platform
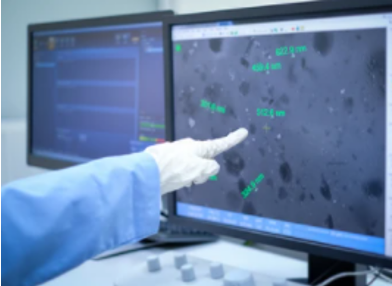
Minimizing foreign particles in drug compounds is a major concern in the pharmaceutical industry, especially for tablet-based and parenteral (injectable) drug compounds. In order to ensure the quality and safety of drugs, it is very important to identify and eliminate particulate contamination in pharmaceutical products quickly and reliably. FDA regulations have stringent requirements for investigating foreign particles, involving their origin and general characteristics, such as shape, size, color, and chemical composition. Therefore, we offer customized and flexible solutions of pharmaceutical foreign particle analysis that contribute to the quality and performance of drug manufacturing.
Pharmaceutical Particulate Contamination
Particulate contamination in pharmaceutical products comes from a wide range of sources, including solutions, packaging, undissolved residues in seals, and so on. The composition of particulate contamination is varied, including metal, glass, and synthetic materials. For example, minimizing particulate contamination is essential for injecting solutions. Contaminating particles of solutions can lead to adverse reactions, including direct and indirect adverse reactions.
- Injection site reactions.
- Particles of a given size can lead to a physiological effect that blocks blood flow.
- As particles spread through the body in the blood, they cause serious complications and organ dysfunction.
- Foreign particles in infusion solutions negatively affect the immune system.
Foreign Particle Analysis at iEM Platform
The iEM Platform is a unique combination of analytical equipment, techniques, and investigative experience. We are capable of performing visual and chemical analyses of particulate contamination. Visual analysis is performed with a high-resolution optical microscope to determine the size, shape, microstructure, and color of the particles. In order to find out root cause analysis (where the foreign particle comes from), chemical analysis of the particles is conducted to determine composition. Scanning electron microscope/ energy-dispersive X-ray spectroscopy (SEM/EDS) is employed for particle chemical analysis and quantification. Our solutions are reliable and professional, enabling efficient identification and elimination of sources of contamination in the pharmaceutical industry.
Why Do You Choose Us?
- Advanced instruments and highly experienced analysts.
- Proper methods of sample isolation and preparation.
- Customized service make customers.
- Complete analytical study reports.
- Our customer service representatives are available 24 hours a day from Monday to Sunday.
Successful drug foreign particle analysis begins with good communication between the analyst and the customer. Feel free to contact us with any questions. We will be delighted to provide you with all the support we can.
- Jack, T., et al. (2010). "Analysis of particulate contaminations of infusion solutions in a pediatric intensive care unit." Intensive care medicine, 36(4), 707-711.

