AAV Vector Characterization Using the iEM Platform
Adeno-associated virus (AAV) is one of the most promising viral vectors for gene therapy and cell therapy. As with any potential therapeutic system, it needs to be thoroughly understood at both in vitro and in vivo levels. With years of AAV vector characterization experience and advanced equipment, we are dedicated to helping to better understand AAV vectors and promote their application for in vitro or in vivo research.
Adeno Associated Virus (AAV) and AAV Vectors
Adenoassociated virus (AAV) is a small replication-deficient parvovirus consisting of a single molecule of linear negative or positive-sense single-stranded DNA that cannot self-replicate. The recombinant adeno-associated virus (rAAV) vector is derived from non-pathogenic wild-type AAV and can infect dividing and non-dividing cells. As one of the important viral vectors for gene therapy and cell therapy, AAV vectors have many advantages, including minimal immunogenicity, having a broad range of tissue tropisms, non-integrating, the potential for long-term gene expression. To date, AAV vectors have been employed in many clinical trials of gene therapy, with satisfactory results, including CFTR, Hemophilia B, arthritis, Parkinson"s diseases, and so on. There are at least eight serologically distinct primary isolates (AV-1–8). Among them, AAV1, AAV2, and AAV9, three serotype capsids have been approved for commercial use.
Adeno Associated Virus (AAV) Vector Characterization at the iEM Platform
Based on our wide range of advanced EM technologies and related analysis techniques, researchers can explore the characteristics of AAV vectors, including
- The integrity of assembled virions and viral aggregates
- The ratio of filled and empty capsids
Intact AAV particles have the required topology to successfully bind to their cognate receptors, enter the cell, cross the cytoplasm, and eventually transport the genome to the nucleus, whereas incompletely assembled particles may lack the ability. Therefore, it is critical to determine the integrity of the assembled virions. Negative stain TEM (nsTEM) is applied to provide detailed information about the AAV particles, including the morphology and integrity of AAV particles as well as the presence of clusters/aggregations.
As one of the most commonly used delivery vectors in gene therapy, AAVs are formed by a closed virus capsid encapsulating a single-stranded DNA. There are three types of capsids produced during the production of AAV vectors, including empty, partially filled, and filled capsids. The ratio of filled and empty capsids is a critical quality attribute requirement for any AAV vector manufacturing process. Cryo-TEM allows the observation of the internal structure of the AAV particles in cryogenic conditions, thus, helps to distinguish between the filling and empty capsids.
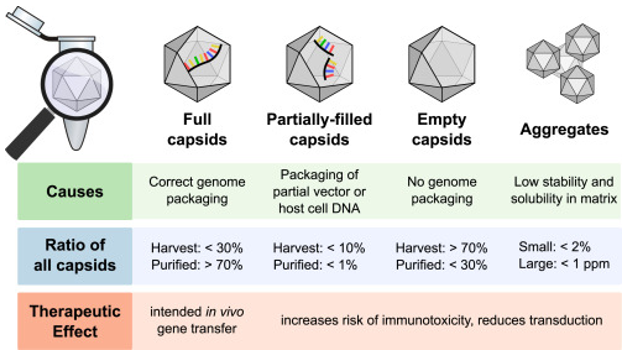 Fig1. Overview of the main types of capsids generated during rAAV production. (Gimpel, A. L., et al, 2021)
Fig1. Overview of the main types of capsids generated during rAAV production. (Gimpel, A. L., et al, 2021)
- The presence of impurities
- The overall capsid stability of AAVs
Empty and partially filled AAV capsids either lack genomic material or contain only the transgenic fragments that give the vector its functionality. They are considered impurities, which may affect the efficacy and safety of AAV vector products. In addition, there are other impurities commonly produced during the manufacturing of AAV vectors, such as mammalian DNA, host cell proteins, and so on.
In order to insights into the structural stability of AAVs, cryogenic electron microscopy (cryo-EM) and functional analysis are applied.
Combined with automated image analysis, our AAV vector characterization can effectively achieve standardized analysis of a large number of capsids. contact us! Talk to our experienced and professional staff to discuss your AAV characterization and analysis needs.
- Dobnik, D.,et al. (2019). "Accurate quantification and characterization of adeno-associated viral vectors." Frontiers in microbiology, 10, 1570.
- Gimpel, A. L., et al. (2021). "Analytical methods for process and product characterization of recombinant adeno-associated virus-based gene therapies." Molecular Therapy-Methods & Clinical Development.

