Liposome Characterization Using the iEM Platform
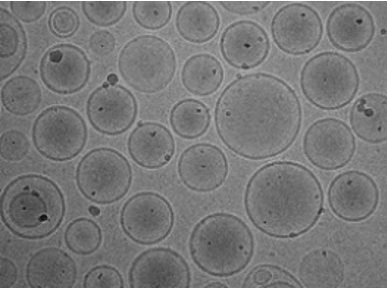
Liposomes are self-assembled and spherical bilayer structures, ranging from tens of nanometers to micrometers. Thanks to the unique structure and composition (an aqueous core surrounded by a hydrophobic membrane), they can be loaded with a wide variety of hydrophobic or hydrophilic molecules for therapeutic purposes. In addition, liposomes are characterized by many advantages, such as low toxicity, easy to be modified, and high capacity for carrying drugs. Therefore, liposomes recently have become one of the most outstanding drug delivery systems. Since its size and drug delivery capacity are closely related to pharmacokinetic and pharmacodynamic parameters of drugs, it is very important to accurately and rapidly monitor the size of liposome vesicles.
Liposomes
Liposomes are typically prepared using materials that contain polar head groups and nonpolar tails, spurring vesicle formation via hydrophobic and hydrophilic interactions. The formulation of liposomes can be further optimized. Cholesterol and PEG- lipid are used to stabilize vesicles and to avoid immune cell attacks, respectively. In addition, liposomes can target specific cells by adding targeting proteins and surface functional ligands on the outer shell of the lipid bilayer.
Liposomes as specialized delivery vehicles have many advantages, including
- Biodegradable, biocompatible, and non-pathogenic.
- Easy and scalable manufacturing.
- Exhibiting high agent-loading efficiency.
- The possible surface modifications (target-oriented).
- Diversity due to various lipid compositions.
Liposome Characterization at iEM Platform
Based on our unique platform, we allow the measurement of liposomes from below 20 nm up to 1 μm with a high degree of accuracy. We support the identification of liposomes, including basic characterization and customized analysis. Sample concentration depends on different factors, such as sample composition. A minimum volume of 60 µL of sample and 2.5 mL of the buffer is required for our analysis.
Basic Characterization
- Liposome size analysis and size distribution.
- Liposome circularity distribution.
- Lamellarity and layer thickness.
Lamellarity means the numbers of lipid bilayers in liposomes which influences the encapsulation efficiency and the drugs release kinetics. Liposome lamellarity becomes an important parameter to characterize for quality control.
Customized Analysis
- Drug encapsulation and loading capacity
Drug encapsulation affects the efficiency of drug release. The encapsulant inside the liposome can be visualized at our iEM Platform. In addition to morphological observations, the ratio of full and empty liposomes and other statistical results can be provided according to customer’s requirements.
- Stability studies
Stability testing is specifically required during liposome characterization. In designing a stability study, physical, chemical and microbial parameters must be considered and evaluated. Aided by our iEM Platforms and experienced experts, researchers can rapidly and extensively investigate liposome stability at different physiological and environmental conditions, such as different temperatures and prolonged storage.
contact us! Talk to our experienced and professional staff to discuss your liposome characterization and analysis needs.
- Large, D. E., et al. (2021). "Liposome composition in drug delivery design, synthesis, characterization, and clinical application." Advanced Drug Delivery Reviews, 113851.

