Lipid Nanoparticle Characterization Using the iEM Platform
Lipid nanoparticle (LNP) systems are currently the lead non-viral delivery systems for enabling the clinical potential of nucleic acids and many other biopharmaceuticals. Similar to liposomes, LNPs belong to lipid-based drug carriers. As versatile drug delivery systems, LNPs provide attribute control of composition, structure, and morphology that can be tailored to each specific drug application (such as drug incorporation and release). Reliable and professional characterization is essential for effective pharmaceutical product development and for regulatory approval. Based on our unique platform, we allow direct and reliable morphological visualization of various LNPs, including SLNs, liquid crystalline nanoparticles, and micellar solutions.
Lipid Nanoparticles
Lipid nanoparticle (LNP) systems have evolved significantly over the past 25 years from their original formulation consisting primarily of phospholipids and cholesterol. Various synthetic and naturally-derived lipids have been employed to form LNPs. As the most commonly used material for non-viral gene delivery systems, LNPs can be divided into several types, including solid lipid nanoparticles (SLNs), nano-emulsions, and micelles. Nowadays, LNPs have been widely applied to deliver nucleic acid payloads such as mRNA, siRNA, plasmid DNA, and antisense oligonucleotides, which have greatly promoted the development and application of genetic drugs.
There are several major advantages of LNPs for gene therapy, including,
- Flexible design and ease of manufacture.
- Low cytotoxicity and immunogenicity.
- High encapsulation efficiency and potent transfection.
- Improved penetration into tissues.
Lipid Nanoparticle Characterization at iEM Platform
The LNP-based drug delivery system holds much promise for many applications, including novel gene therapies, oncology, and effective vaccines. In R&D, production, and quality control, it is important to develop platform technologies that characterize and quantify LNP attributes in terms of size, shape, and surface properties. In this respect, cryo-transmission electron microscopy (cryo-TEM) as an indispensable characterization technique, has been employed to provide detailed morphological characterization and the internal nanostructure of LNPs.
Advantages of Cryo-TEM Investigations of LNPs
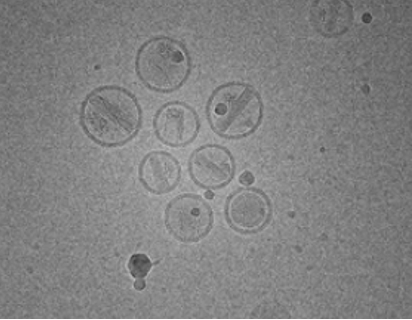
- Preserve the sample near its native state and avoid any fixation of the sample
(It is important to note that the sample fixation process may lead to lipid extraction, resulting in artifacts). - Provide high-resolution images suitable for studying samples with multiple structures of different shapes and sizes.
- Enable to capture metastable and short-lived intermediate species, promoting a better understanding of self-assembly, dynamic phenomena, and phase transition mechanisms in complex lipid-based structures.
Creative Biostructure’ solutions for analyzing critical LNP quality attributes, including,
- Average size and detailed size distribution.
- Evaluation of sample polydispersity.
- The internal nanostructure of LNPs.
- Drug encapsulation and loading capacity.
- Stability studies of LNPs.
The iEM Platform is a powerful and versatile system for the analysis and characterization of nanoparticles, viral-based biotherapeutics, and other applications in biotherapeutic development and production. Based on our iEM Platform, we are able to provide an accurate and reliable characterization of LNPs. contact us! Talk to our experienced and professional staff to discuss your LNP characterization and analysis needs.
- Helvig, S., et al. (2015). "Recent advances in cryo-TEM imaging of soft lipid nanoparticles." Aims Biophysics, 2(2), 116-130.
- Cullis, P. R., & Hope, M. J. (2017). "Lipid nanoparticle systems for enabling gene therapies." Molecular Therapy, 25(7), 1467-1475.

